[vc_row][vc_column][vc_column_text]
Desert Iguana (Dipsosaurus dorsalis)
[/vc_column_text][gap size=”12px” id=”” class=”” style=””][/vc_column][/vc_row][vc_row][vc_column width=”1/2″][vc_single_image image=”1263″ img_size=”large” alignment=”center” style=”vc_box_rounded”][vc_column_text]Desert Iguana, Yuma Co., AZ. Photo by Jim Rorabaugh[/vc_column_text][/vc_column][vc_column width=”1/2″][vc_row_inner][vc_column_inner width=”1/2″][vc_single_image image=”1667″ img_size=”medium” alignment=”center” onclick=”img_link_large”][vc_column_text]Desert Iguana, Pima County, AZ. Photo by Jim Rorabaugh[/vc_column_text][gap size=”12px” id=”” class=”” style=””][/vc_column_inner][vc_column_inner width=”1/2″][vc_single_image image=”1665″ img_size=”medium” alignment=”center” style=”vc_box_rounded” onclick=”img_link_large”][vc_column_text]Desert Iguana at burrow entrance. Yuma County, AZ. Photo by Jim Rorabaugh[/vc_column_text][gap size=”12px” id=”” class=”” style=””][/vc_column_inner][/vc_row_inner][vc_row_inner][vc_column_inner width=”1/2″][vc_single_image image=”1675″ img_size=”medium” alignment=”center” style=”vc_box_rounded” onclick=”img_link_large”][vc_column_text]Desert Iguana with White-lined Sphinx Moth, Yuma County, AZ. Photo by Jim Rorabaugh[/vc_column_text][gap size=”12px” id=”” class=”” style=””][/vc_column_inner][vc_column_inner width=”1/2″][vc_single_image image=”1670″ img_size=”medium” alignment=”center” style=”vc_box_rounded” onclick=”img_link_large”][vc_column_text]Desert Iguana at burrow exit. Yuma County, AZ. Photo by Jim Rorabaugh[/vc_column_text][gap size=”12px” id=”” class=”” style=””][/vc_column_inner][/vc_row_inner][vc_row_inner][vc_column_inner width=”1/2″][vc_single_image image=”1658″ img_size=”medium” alignment=”center” style=”vc_box_rounded” onclick=”img_link_large”][vc_column_text]Juvenile Desert Iguana impaled on a creosote by a Loggerhead Shrike. Imperial Co., CA. Photo by Jim Rorabaugh[/vc_column_text][gap size=”12px” id=”” class=”” style=””][/vc_column_inner][vc_column_inner width=”1/2″][vc_single_image image=”1663″ img_size=”medium” alignment=”center” style=”vc_box_rounded” onclick=”img_link_large”][vc_column_text]Sidewinder preying on a Desert Iguana, Algodones Dunes, SE CA. Photo by Jim Rorabaugh[/vc_column_text][gap size=”12px” id=”” class=”” style=””][/vc_column_inner][/vc_row_inner][vc_row_inner][vc_column_inner width=”1/2″][vc_single_image image=”1672″ img_size=”medium” alignment=”center” style=”vc_box_rounded” onclick=”img_link_large”][vc_column_text]Long-nosed Leopard Lizard preying on Desert Iguana, Gran Desierto, Sonora. Photo by Jim Rorabaugh[/vc_column_text][gap size=”12px” id=”” class=”” style=””][/vc_column_inner][vc_column_inner width=”1/2″][vc_single_image image=”1671″ img_size=”medium” alignment=”center” style=”vc_box_rounded” onclick=”img_link_large”][vc_column_text]Juevnile Desert Iguana in a Croton wigginsii, Cabeza Prieta NWR, AZ. Photo by Jim Rorabaugh[/vc_column_text][gap size=”12px” id=”” class=”” style=””][/vc_column_inner][/vc_row_inner][gap size=”12px” id=”” class=”” style=””][vc_row_inner][vc_column_inner width=”1/2″][vc_single_image image=”1666″ img_size=”medium” alignment=”center” style=”vc_box_rounded” onclick=”img_link_large”][vc_column_text]Desert Iguana. Picture Rocks, Avra Valley, AZ. Photo by Douglas Moore[/vc_column_text][gap size=”12px” id=”” class=”” style=””][/vc_column_inner][vc_column_inner width=”1/2″][vc_single_image image=”1664″ img_size=”medium” alignment=”center” style=”vc_box_rounded” onclick=”img_link_large”][vc_column_text]Desert Iguana near Pueblo de Alamos, Sonora. Photo by Jim Rorabaugh.[/vc_column_text][gap size=”12px” id=”” class=”” style=””][/vc_column_inner][/vc_row_inner][/vc_column][/vc_row][vc_row][vc_column width=”1/6″][/vc_column][vc_column width=”2/3″][vc_column_text]
Description
The June sun rises like a ball of fire scorching the parched creosote flats of the Sonoran Desert. Soon cicadas commence their song of the dry, and Common Side-blotched Lizards (Uta stansburiana) seek refuge in cool, moist underground retreats. By mid-morning Tiger Whiptails (Aspidoscelis tigris) confine their jerky foraging to shade patches, and Zebra-tailed Lizards (Callisaurus draconoides) raise their toes off the searing desert pavement. But the large, pale “thirsty lizard” (Dipsosaurus dorsalis, Desert Iguana, Figure 1) is just issuing forth from its burrow to bask in the hot sun. When its body temperature reaches 40°C (104°F) the herbivore climbs into a Creosotebush to browse on the few remaining yellow blossoms. Later it switches tactics, facing into the sun to minimize the surface area of its body that is exposed to the rays. The lizard remains active into the heat of the early afternoon, and its body temperature may reach 46.4°C (116°F), the highest recorded for a reptile.
 Figure 1. Desert Iguana (Dipsosaurus dorsalis), Yuma Co., Arizona. Photo by Erik F. Enderson.
Figure 1. Desert Iguana (Dipsosaurus dorsalis), Yuma Co., Arizona. Photo by Erik F. Enderson.
The importance of temperature tolerance in the ecology and evolution of desert reptiles was articulated by Ray Cowles (1940a, 1940b). In 1944, the theme of behavioral thermal regulation was developed in grand style by Cowles and his former graduate student Chuck Bogert (see Pough 1974, Myers and Zweifel 1993, Mole and Rubio 2006), much to the dismay of Chuck Lowe, who also was a student of Cowles. After Bogert (1949) introduced the Schultheis rapid-reading cloacal thermometer, “noose-em and goose-em” became a herpetological cottage industry, and Lowe brought the zeal for thermal ecology to the “Creosotebush League” when he joined the faculty of the University of Arizona in 1950 (see Rosen 2004). But the stellar role of the Desert Iguana in reptile thermal biology remained little appreciated until Ken Norris, also a former student of Cowles and later faculty member at UCLA, had the fortitude to conduct and publish the first thorough study of the ecology of this thermophilic herbivorous lizard (Norris 1953).
He traveled throughout the species’ range from the Mohave Desert to Sinaloa and the cape of Baja California Sur, but his work was centered in the Sonoran Desert of southern California where he firmly established an understanding of the basic features of the life of the Desert Iguana. Knowledge of its ecology has been filled out by a large number of subsequent workers in southern California, particularly Mayhew (1965, 1971), McGinnis and Dickson (1967), Pianka (1971), Berke and Heath (1975), Krekorian (1976, 1977, 1983, 1984), Muth (1977, 1980), Mautz and Nagy (1987), and Howland (1988). As is the case for most lizards, little published research exists for Arizona populations, and the effect of the monsoons and summer annuals on its ecology can be estimated only by a few observations in Baja California Sur (Asplund 1967, Grismer 2002). Parker (1972) presented information, primarily on hatchlings, gathered incidental to his study of the Western Banded Gecko (Coleonyx variegatus) in South Mountain Park, Maricopa Co., Arizona, and a useful species summary and bibliography were provided by Hulse (1988, 1992).
Norris (1953) pointed out the wide spectrum of habitats occupied by the Desert Iguana, but emphasized that the species is closely associated with the Creosotebush (Larrea tridentata) in the Southwestern deserts where the flowers comprise a major part of the diet of this herbivorous reptile. The long tail facilitates climbing in the bushes to forage or to escape the extreme heat of the desert floor. The mottled pattern of the dorsal surface is well camouflaged in the dappled sunlight beneath the creosote (Figure 2), where the lizards’ burrows are usually located. The burrows play an important role in the ecology of the Desert Iguana. Some may have been dug originally by Round-tailed Ground Squirrels (Xerospermophilus tereticaudus) or Desert Kangaroo Rats (Dipodomys deserti), but Norris found that most were quite shallow and appeared to have been excavated by the lizard, particularly in sandy soils. They usually contained a distinct chamber just a few inches below the surface, and the entrances were seen to have been plugged by sand. Smaller Desert Iguanas occupied smaller burrows, additional evidence that these lizards dig their own burrows. Some burrows are inhabited by more than one lizard (Krekorian 1976), and individuals have been observed to use more than one burrow (Howland 1988).
Figure 2. Desert Iguana (Dipsosaurus dorsalis) in dappled sunlight beneath a Creosotebush (Larrea tridentata), Yuma Co., Arizona. Photo by Erik F. Enderson.
Jeff Howland (pers. comm.) observed an adult dig its burrow. It started by scraping out a round hollow about 30 cm (1 ft) in diameter and 1-2 cm (0.4-0.8 in) deep at the center. Then it began digging a burrow in the middle. Within about an hour, it had a burrow deep enough to easily allow the lizard to get under ground, and the hollow had been filled to ground level by soil excavated from the burrow. By this time, it was early afternoon and the lizard then went into the burrow and back-filled the entrance, as they often do at the end of the daily activity period, leaving the burrow and excavation area barely visible. The burrow is defended with an assertion display given by both sexes, and a more vigorous challenge display performed mostly by males. Like those of most Southwestern iguanid lizards, the display involves pushups, lateral compression of the body, and extension of the gular and ventral surfaces, but it is species specific in format. In the most aggressive display, the challenger pins his opponent against his body and inflicts a violent, audible tail slap (Carpenter 1961).
Unlike most other lizards, the home range of females (1,558 m2 [0.38 acre]) is nearly identical in size to that of males (1,462 m2 [0.36 acre]), and this probably reflects the near lack of size sexual dimorphism in the species (Krekorian 1976). Male home ranges overlap extensively, whereas those of females do not. Alberts (1989) has demonstrated that territories are marked by males with waxy secretions from the femoral pores that absorb UV light that is visible to Desert Iguanas. Gier found that males who establish territories around large shade plants have access to a greater number of females (Pianka and Vitt 2003).
Estimates of population densities vary considerably among localities and years. Krekorian (1983, 1984) found 300-700 individuals per hectare in the Coachella Valley, Riverside Co., California, whereas 145 km (90 mi) to the east in the more arid Chuckwalla Valley, Howland (1988) found only 60 lizards per hectare, presumably due to lower food availability. The daily activity pattern of Desert Iguanas differs from that of most other Sonoran Desert lizards in that it is unimodal, centered in the mid to late morning. In April most activity occurs from 0930 to 1400 hrs, whereas in July it centers on 0900 to 1300 hrs (Howland 1988). Not all lizards are active each day, and some individuals were not observed out for several days (Krekorian 1976). Average body temperatures of foraging lizards range from 41.0 to 43.8°C (105.8 to 110.8°F; Norris 1953, McGinnis and Dickson 1967, Krekorian 1976, Howland 1988). The highest body temperature recorded was 46.4°C (115.5°F), taken with a cloacal thermometer at 1030 on 10 July 1948 by Norris (1953), and this appears to be a global record of voluntary thermal maximum for reptiles (summary in Brattstrom 1965). The figure is consistent with the field data of Howland (1988) in which the maximum cloacal temperature was 46.0°C (114.8°F) and of McGinnis and Dickson (1967) who found deep body temperatures ranging to 45.7°C (114.3°F). These voluntary maxima are just below the critical thermal maximum (47.5°C [117.5°F]) and lethal temperature (50.5°C [122.9°F]) for the species (Cowles and Bogert 1944), comprising an often cited example of “life on the edge” in the thermal ecology of desert reptiles.
The activity season for adult Desert Iguanas in the Sonoran Desert extends from March to September, but juveniles may be active as early as February and as late as October (Parker 1972, Howland 1988). In the Chuckwalla Valley, females cease activity shortly after egg laying, suggesting the possibility that egg brooding may occur during this period (Jeff Howland, pers. comm.). A small percentage of the females become active again just before the hatchlings appear in August, whereas male activity gradually decreases in August though September (Howland 1988).
Courtship and mating were observed in enclosures by Carpenter (1961). The males approach females with a rapid head bob. If she does not retreat, he bites her shoulder and brings his body on top of hers and attempts cloacal apposition (Carpenter 1961). Jeff Howland (pers. comm.) observed the entire sequence of courtship and mating several times in the Chuckwalla Valley (Figure 3). In addition he observed a strange behavior involving abdominal contact similar that described by Carpenter (1961) for lizards in an enclosure. This consisted of young, probably immature, females crawling under adult males and flipping over on their backs in the process. The males arched their backs a little to allow passage. In each case, the young female passed back and forth under the male several times, and then the interaction ended. The males remained passive during the activity.
Figure 3. Desert Iguanas (Dipsosaurus dorsalis) mating in the Chuckwalla Valley, Riverside Co., California. Photo by Jeffrey M. Howland.
One clutch containing 2-8 (usually 3 to 5) eggs is deposited during late May or early June (Norris 1953, Howland 1988), but perhaps as late as August (Mayhew 1971). Hatchlings emerge during a four week period centered in August (Howland 1988, Parker 1972).
Growth of Desert Iguanas is remarkably slow and varies with food availability (Howland 1988). In Arizona, Parker (1972) estimated an average growth rate for hatchlings of ca 20 mm/year (0.8 in/year). Age at maturity (105 mm to 120 mm SVL [4.1 to 4.7 in]) has been calculated at 31 to 54 months (Mayhew 1971, Parker 1972, Muth 1977, Mautz and Nagy 1987, Howland 1988). This is a long-lived lizard with a longevity of over 7 years in the wild (Krekorian 1984) and over 14 years in captivity (Bowler 1977). Survivorship is also high, averaging 50% to 60% per year in both adults and subadults (Parker 1972, Krekorian 1984, Howland 1988).
Avoidance of predation may be one benefit of the late morning-early afternoon activity period of the Desert Iguana (Pianka and Vitt 2003). Howland (1988) witnessed five predation events on Desert Iguanas. Two of these involved moderately sized Coachwhips (Coluber flagellum) that separately preyed on an adult male and an adult female Dipsosaurus. One hatchling was observed to be taken by a large Tiger Whiptail (Aspidoscelis tigris) and another by a Loggerhead Shrike (Lanius ludovicianus). An adult female Long-nosed Leopard Lizard (Gambelia wislizenii) was observed to prey on an adult female Desert Iguana (Figure 4). Eight to nine days previous the Desert Iguana was 121 mm SVL (4.8 in) and 53.3 g (1.9 oz), and the leopard lizard was 146 mm SVL (5.7 in) and 80.8 g (2.9 oz). The leopard lizard consumed the entire Desert Iguana except of a 97-mm (1.5-g) tail tip that was broken off by biting and head shaking. Immediately after the predation event the leopard lizard weighed 127.2 g (4.5 oz; Jeff Howland, pers. comm). Diet has been investigated by Norris (1953) and Mautz and Nagy (1987) in the Coachella Valley and by Howland (1988) in Chuckwalla Valley. All three studies found that the flowers of Creosotebush formed the staple during the spring. In the Coachella, the spring diet also included leaves of Palmer’s Coldenia (Coldenia palmeri) and flowers of Desert Palafox (Palafoxia linearis) and White Bursage (Ambrosia dumosa). The autumn diet centered on leaves of Coldenia palmeri, Desert Dicoria (Dicoria canescens), and Indigo Bush (Dalea schottii), and the flowers and leaves Emory Dalea (Dalea emoryi). In the Chuckwalla Valley, the spring diet also included flowers of Coulter’s Lupine (Lupinus sparsiflorus), Fremont’s Pincushion (Chaenactis fremonti), and Ironwood (Olnea tesota). During the rest of the year the leaves, seeds, and dried flowers of ironwood were eaten. Except for Creosotebush flowers in the spring, there is no overlap in the main diet items at the two localities, and the Desert Iguana appears to be capable of subsisting on a wide variety of plants depending on the vegetation present in its habitat.
Figure 4. Long-nosed Leopard Lizard (Gambelia wislizenii) preying on a Desert Iguana (Dipsosaurus dorsalis) in the Chuckwalla Valley, Riverside Co., California. Photo by Jeffrey M. Howland.
In southern California, a small amount of animal material (1-3%) was consumed, including insects, rodent feces, and even other Desert Iguanas (Norris 1953, Mautz and Nagy 1987, Howland 1988). Remarkably, arthropods were found to comprise 41-68% of the stomach contents of Desert Iguanas in the tropical deciduous forest of Cape Region of Baja California Sur by Asplund (1967), and Galina-Tesaro found that on the La Paz plains the species consumed 100% lepidopteran larvae during the late-summer rains (Grismer 2002). Thus, herbivory does not appear to be a fixed ecological attribute, and the species may be primarily insectivorous at more tropical latitudes.
From a physiological viewpoint, the herbivory of the Desert Iguana is of special interest. Pough (1973) found that a diet consisting predominately of plant material occurs mostly in lizard species that have an adult body weight of greater than 100 g (3.5 oz). Adult Dipsosaurus weigh between 25 g and 75 g (0.9 to 2.6 oz; Minnich 1971), and even hatchlings are primarily herbivorous (Mautz and Nagy 1987). However, since Pough’s (1973) paper many exceptions have been identified to the necessity of large body size to support herbivory (summaries in Greene 1982, Pianka and Vitt 2003). These include the switch to partial herbivory by Coachella Fringe-toed Lizards (Uma inornata) in times of abundant flowers (Durtsche 1992). Iverson (1980, 1982) examined the gut structure of iguanines and determined that Dipsosaurus is the least specialized for digestion of plant material. The high preferred body temperature of the Desert Iguana may facilitate digestion by this relatively small iguanine herbivore (Mautz and Nagy 1987). Pianka and Vitt (2003) suggested that the consumption of mammal feces (Norris 1953, Mautz and Nagy 1987) may help supply the micro-organisms to the digestive tract needed for processing plant material.
On the basis of phylogeny, herbivory is expected in Dipsosaurus. The genus is a member of a natural group (monophyletic clade) that includes about 30 species in seven (Frost and Etheridge 1989) or eight (Etheridge 1982) genera that are large bodied, herbivorous, and have a mid-dorsal crest (reduced in size in Dipsosaurus and absent in Sauromalus, chuckwallas). The clade is known as the Iguanidae in the taxonomic arrangement of Frost and Etheridge (1989) or as the Iguaninae in that of Schulte et al. (2003). Herbivory and large body size are thus two shared and probably derived features of iguanine lizards. As an adaptation to arid environments, the Desert Iguana has either retained the primitive ancestral condition of small size (relative to other iguanines) or has undergone an evolutionary reversal (i.e., re-evolved small size), but the species has maintained herbivory perhaps via behaviors that achieve uniquely high body temperatures.
The position of the Desert Iguana within the Iguaninae is not well resolved. On the basis of morphology Etheridge and de Queiroz (1988) found it to be a relatively early branch of the subfamily and to be part of a basal trichotomy composed of (1) the Desert Iguana (Dipsosaurus); (2) the Fiji iguanas (Brachylophus); and (3) all other iguanines. In a combined analysis of morphology and DNA sequences, Sites et al. (1996) found it to be the earliest branch within iguanines, with the possible exception of the Fiji iguanas. Thus, Dipsosaurus dorsalis appears to represent an ancient lineage that is now restricted to the Mohave and Sonoran deserts and adjacent thornscrub and tropical deciduous forest. Its association with the Creosote-bush must be of relatively recent origin as Larrea did not arrive in North America until sometime in the Pleistocene (Van Devender 1990).
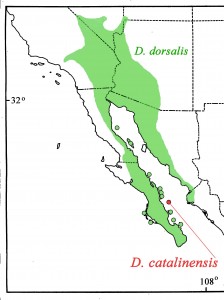
The Desert Iguana ranges in Baja California Sur from Cabo San Lucas to Laguna San Ignacio, north of which it becomes restricted to the gulf side of the peninsula (Grismer 2002); it extends into the Sonoran Desert of Baja California, southern California, western Arizona, Sonora, and northern Sinaloa, and into the Mohave Desert of southern California, southern Nevada, northwestern Arizona, and extreme southwestern Utah (Figure 5). The northern limit of the species appears to be correlated with that of the Creosotebush (Larrea tridentata; Norris 1953). In Sinaloa it is known south to near Topolobompo (25°33’N; Hardy and McDiarmid 1969). It occurs on Islas Ángel de la Guardia, Carmen, Cerralvo, Coronados, Espíritu Santo, Monserrate, Partida Sur, San José, San Luis, and San Marcos in the Gulf of California, and on Islas Magdalena and Santa Margarita in the Pacific (Grismer 2002). Its absence on Isla Tiburón is enigmatic.
Figure 5. Geographic distribution of the Desert Iguana (Dipsosaurus dorsalis; green) and the Isla Santa Catalina Desert Iguana (Dipsosaurus catalinensis; red).
The easternmost known localities for the species in Arizona are Snyder Hill, Rillito on the Santa Cruz River, Pima Co.; Florence, Pinal Co.; Blue Point on the Salt River, Fort McDowell, Cave Creek, Maricopa Co.; 16 km (10 mi) NE Aguila, Yavapai Co.; Burro Creek, Big Sandy River, and Virgin River, Mohave Co. The locality of Audley, Yavapai Co., listed for USNM 45003, collected in 1909, may represent a rail shipping station rather than the point of collection.
The distribution in the Tucson area is curious. The Avra Valley is often considered to be the eastern limit of the species in southern Arizona. But previously it may have extended a short distance east around the ends of the Tucson Mountains as is indicated by UAZ 15328, found dead on the road at the junction of Ina and Silverbell Roads by Oscar Soule on 26 July 1966 (32.3374198°N, 111.0877314°W) and by UAZ 28748 listed as having been collected at Snyder Hill, Ajo Hwy, 14 km (8.7 mi) W and 7.9 km (4.9 mi) S of Tucson on 13 Apr 1968 by James DeWeese (32.1576675°N, 111.1132820°W). As pointed out by Rosen (2007) the species penetrates farther into the Arizona Upland Desert than most species centered in the creosote flats of the Colorado and Mohave deserts, and he records a Desert Iguana at 1128 m (3700 ft) in desert grassland in Ban Thak Pass.
No range-wide studies of geographic variation in morphology or DNA sequences have been conducted. Lamb et al. (1992) examined mitochondrial haplotypes of the Desert Iguana in western Arizona and southeastern California and uncovered significant geographic structure, but this did not reflect a break at the Colorado River as found in the Desert Tortoise (Gopherus agassizii; Lamb et al. 1989).
Hulse (1992) mapped five subspecies of the Desert Iguana. Grismer et al. (1994) found no consistent morphological differences between the two peninsular subspecies and placed D. d. lucasensis in the synonymy of D. d. dorsalis. Similarly, Grismer (1999) found that D. d. carmenensis could not be differentiated from peninsular populations and synonymyzed it with D. d. dorsalis. He found D. catalinensis to have a unique darkly suffused gular surface (see Figure 4.12 in Grismer 2002) and considered it to represent a distinct species. Burger and Hensley (1949) redefined Dipsosaurus d. sonoriensis (Allen 1933) on the basis of color pattern (rather than scalation), and the subspecies was recognized by Bogert and Oliver (1945), Langebartel and Smith (1954), and Hardy and McDiarmid (1969); its status has not been appraised in more recent times. Thus, the genus contains three taxa: Isla Santa Catalina Desert Iguana, D. catalinensis Van Denburgh (1922); Northern Desert Iguana, D. dorsalis dorsalis (Baird and Girard 1852), and Sonoran Desert Iguana, D. dorsalis sonoriensis Allen (1933; status unknown).
Baird and Girard (1852) described Crotaphytus dorsalis from the “Desert of Colorado, Cal.”, and Smith and Taylor (1950) restricted the type locality to “Winterhaven (=Fort Yuma), Imperial County.” Hallowell (1854) established the genus Dipsosaurus, which he amended to Dipsosaurus in 1859. The Comcáac (Seri) consider the Desert Iguana to be extremely dangerous (Felger 1990, Nabhan 2003), and its Comcáac name translates as “thing, that if it bites, prevents from arriving to home camp.” A legend relates how a traveler who failed to arrive at his home camp was found dead in an arroyo with a Desert Iguana nearby. The pale coloration of the species may contribute to the fear in which it is held. The Tohono O’odham are said traditionally to have eaten Desert Iguanas.
The generic name Dipsosaurus is derived from dipsa (thirsty) and sauros (lizard). The species name dorsalis is derived from dorsum (back) and refers to the enlarged row of vertebral scales.
Acknowledgements.
I thank Jeff Howland for generously providing photographs, personal observations, and helpful suggestions on a previous draft of this paper; Erik Enderson for contributing photographs; Oscar Soule and George Bradley for information; and Kit Bezy and Kate Bolles for helpful suggestions on the figures and text.
Literature Cited
Alberts, A.C. 1989. Ultraviolet visual sensitivity in Desert Iguanas: implications for pheromone detection. Animal Behaviour 144:551-564.
Allen, M.J. 1933. Report on a collection of amphibians and reptiles from Sonora, Mexico, with the description of a new lizard. Occasional Papers, Museum of Zoology, University of Michigan 259:1-15.
Asplund, K.K. 1967. Ecology of lizards in the relictual cape flora, Baja California. American Midland Naturalist 77:462-475.
Baird, S.F., and C. Girard. 1852. Characteristics of some new reptiles in the museum of the Smithsonian Institution, Part II. Proceedings of the Academy of Natural Sciences Philadelphia 6:125-129.
Bogert, C.M. 1949. Thermoregulation in reptiles, a factor in evolution. Evolution 3:195-211.
Bogert, C.M., and J.A. Oliver. 1945. A preliminary analysis of the herpetofauna of Sonora. Bulletin of the American Museum of Natural History 83:301-425.
Bowler, J.K. 1977. Longevity of reptiles and amphibians in North American collections. Herpetological Circular 6:1-32.
Burger, W.L., and M.M. Hensley. 1949. Notes on a collection of reptiles and amphibians from northeastern Sonora. Natural History Miscellenea, Chicago Academy of Sciences 35:1-6.
Berke, L.L., and J.E. Heath. 1975. An analysis of behavioral thermoregulation in the lizard Dipsosaurus dorsalis. Journal of Thermal Biology 1:15-22.
Brattstrom, B.H. 1965. Body temperatures of reptiles. American Midland Naturalist 73:376-442.
Carpenter, C.C. 1961. Patterns of social behavior in the Desert Iguana, Dipsosaurus dorsalis. Copeia 1961:396-405.
Cowles, R.B. 1940a. Observations on the winter activities of desert reptiles. Ecology 22:125-140.
Cowles, R.B. 1940b. Additional implications of reptilian sensitivity to high emperatures. American Naturalist 74:542-561.
Cowles, R.B., and C.M. Bogert. 1944. A preliminary study of the thermal requirements of desert reptiles. Bulletin of the American Museum of Natural History 83:261-296.
Durtsche, R.D. 1992. Feeding time strategies of the fringe-toed lizard, Uma inornata, during breeding and non-breeding seasons. Oecologia 89:85-89.
Etheridge, R.E. 1982. Checklist of iguanine and Malagasy iguanid lizards. Pages 60-76 in: G.M. Burghartd and A. S. Rand, Iguanas of the World. Noyes Publications, Park Ridge, New Jersey.
Etheridge, R., and K. de Queiroz 1988. A phylogeny of the Iguanidae. Pages 7-34 in: Phylogenetic Relationships of the Lizard Families. Stanford University Press, Stanford, CA.
Felger, R.S. 1990. The Seri Indians and their herpetofauna. Sonoran Herpetologist 3:41-44.
Frost, D.R., and R. Etheridge. 1989. A phylogenetic analysis and taxonomy of iguanian lizards. Miscellaneous Publications, Museum of Natural History, University of Kansas 81:1-65.
Greene, H. W. 1982. Dietary and phenotypic diversity in lizards: why are some organisms specialized? Pages 107-128 in: D. Mossakowski and G. Roth (editors). Environmental Adaptation and Evolution: a Theoretical and Empirical Approach. G. Fischer-Verlag, Stuttgart.
Grismer, L.L. 1999. An evolutionary classification of reptiles on islands in the Gulf of California, Mexico. Herpetologica 55:446-469.
Grismer, L.L. 2002. Amphibians and Reptiles of Baja California. University of California Press, Berkeley.
Grismer, L.L., J.A. McGuire, and B.D. Hollingsworth. 1994. A report on the herpetofauna of Vizcaíno Peninsula, with a discussion of its biogeographic and taxonomic implications. Bulletin of the Southern California Academy of Sciences 93:45-80.
Hallowell, E. 1854. Descriptions of new reptiles from California. Proceedings of the Academy of Natural Sciences Philadelphia 7:91-97.
Hallowell, E. 1859. Report upon the reptiles collected on the survey. Pages 1-27 in: R.S. Williamson (editor). Volume 10. Report of explorations in California.
Hardy L.M., and R.W. McDiarmid. 1969. The amphibians and reptiles of Sinaloa, Mexico. University of Kansas Publications of the Museum of Natural History 18:39-215.
Howland, J.M. 1988. Natural history of the Desert Iguana Dipsosaurus dorsalis. Pages 51-59 in: H.F. De Lisle, P.R. Brown, B. Kaufman, and B.M. McGurty (editors). Proceedings of the Conference on California Herpetology. Southwestern Herpetologists Society Special Publication 4:1-142.
Hulse, A.C. 1988. A bibliography of Dipsosaurus dorsalis. Smithsonian Herpetological Information Service 74:1-25.
Hulse, A.C. 1992. Dipsosaurus, D. dorsalis. Catalogue of American Amphibians and Reptiles 542:1-6.
Iverson, J.B. 1980. Colic modifications in iguanine lizards. Journal of Morphology 163:79-83.
Iverson, J.B. 1982. Adaptations to herbivory in iguanine lizards. Pages 60-76 in: G.M. Burghart, and A.S. Rand (editors). Iguanas of the World. Noyes Publications, Park Ridge, New Jersey.
Krekorian, C.O. 1976. Home range size and overlap and their relationship to food abundance in the Desert Iguana, Dipsosaurus dorsalis. Herpetologica 32:405-412.
Krekorian, C.O. 1977. Homing in the Desert Iguana, Dipsosaurus dorsalis. Herpetologica 33:123-127.
Krekorian, C.O. 1983. Population density of the Desert Iguana, Dipsosaurus dorsalis (Reptilia: Iguanidae) in southern California. Copeia 1983:268-271.
Krekorian, C.O. 1984. Life history of the Desert Iguana, Dipsosaurus dorsalis. Journal of Herpetology 23:267-273.
Lamb, T., J.C. Avise, and J.W. Gibbons. 1989. Phylogeographic patterns in mitochondrial DNA of the desert tortoise (Xerobates agassizii), and evolutionary relationships among the North American tortoises. Evolution 43:76- 87.
Lamb, T., T.R. Jones, and J.C. Avise. 1992. Phylogeographic histories of representative herpetofauna of the southwestern U.S.: mitochondrial DNA variation in the Desert Iguana (Dipsosaurus dorsalis) and the chuckwalla (Sauromalus obesus). Journal of Evolutionary Biology 5:465-480.
Langebartel, D.A., and H.M. Smith. 1954. Summary of the Norris collection of reptiles and amphibians from Sonora. Herpetologica 10:125-136.
Mautz, W.J., and K.A. Nagy. 1987. Ontogenetic changes in diet, field metabolic rate, and water flux in the herbivorous lizard Dipsosaurus dorsalis. Physiological Zoology 60:640-658.
Mayhew, W.W. 1965. Growth response to photoperiodic stimulation in the lizard Dipsosaurus dorsalis. Comparative Biochemistry and Physiology 14:209-216.
Mayhew, W.W. 1971. Reproduction in the desert lizard, Dipsosaurus dorsalis. Herpetologica 27:57-77.
McGinnis, S.M., and L.L. Dickson. 1967. Thermoregulation in the Desert Iguana Dipsosaurus dorsalis. Science 156:1757-1759.
Minnich, J.E. 1971. Seasonal variation in weight-length relationships and fat body size in the Desert Iguana, Dipsosaurus dorsalis. Copeia 1971:359-362.
Moll, E.O., and M. Rubio. 2006. Patronyms of the Pioneer West. Bogertophis subocularis (Brown, 1901), Trans-Pecos Ratsnake. Sonoran Herpetologist 19:110-114.
Muth, A. 1977. Eggs and hatchlings of captive Dipsosaurus dorsalis. Copeia 1977:189-190
Muth, A. 1980. Physiological ecology of Desert Iguana (Dipsosaurus dorsalis) eggs: temperature and water relations. Ecology 61:1335-1343.
Myers, C.W., and R.G. Zweifel. 1993. Biographical sketch and bibliography of Charles Mitchill Bogert, 1908-1992. Herpetologica 49:133-146.
Nabhan, G.P. 2003. Singing the Turtles to Sea. The Comcáac (Seri) Art and Science of Reptiles. University of California Press, Berkeley.
Norris, K.S. 1953. The ecology of the Desert Iguana Dipsosaurus dorsalis. Ecology 34:265-287.
Parker. W.S. 1972. Notes on Dipsosaurus dorsalis in Arizona. Herpetologica 28:226-229.
Pianka, E.R. 1971. Comparative ecology of two lizards. Copeia 1971:129-138.
Pianka, E.R., and L.J. Vitt. 2003. Lizards. Windows to the Evolution of Diversity. University of California Press, Berkeley.
Pough, F.H. 1973. Lizard energetics and diet. Ecology 54:837-844.
Pough, F.H. 1974. Preface. Pages i-iv in: R. B. Cowles and C. M. Bogert (editors). A preliminary study of the thermal requirements of desert reptiles. SSAR reprint 1974.
Rosen, P.C. 2004. Charles Herbert Lowe, Jr. 1920-2002. Copeia 2004:961-972.
Rosen, P.C. 2007. Reptiles and amphibians of arid southwestern Arizona and northwestern Sonora. Pages 310-337 in: R.S. Felger and B. Broyles (editors). Dry Borders. Great Natural Reserves of the Sonoran Desert. University of Utah Press, Salt Lake City.
Schulte, J.A. II, J.P. Valadares, and A. Larson. 2003. Phylogenetic relationships within Iguanidae using molecular and morphological data and a phylogenetic taxonomy of iguanian lizards. Herpetologica 59:399-419.
Sites, J.W. Jr., S.K. Davis, T. Guerra, J.B. Iverson, and H.L. Schnell. 1996. Character congruence and phylogenetic signal in molecular and morphological data sets: a case study in the living iguanas (Squamata, Iguanidae). Molecular Biology and Evolution 13:1087-1105.
Smith, H.M., and E.H. Taylor. 1950. An annotated checklist and key to the reptiles of Mexico, exclusive of snakes. Smithsonian Institution United States National Museum Bulletin 199:1-253.
Van Denburgh, J. 1922. The Reptiles of Western North America. Volume I Lizards. Occasional Papers of the California Academy of Sciences 10:1-611.
Van Devender, T.R. 1990. Late Quaternary vegetation and climate of the Sonoran Desert, United States and Mexico. Pages 134-163 in: J.L. Betancourt, T.R. Van Devender, and P.S. Martin (editors). Packrat Middens: the Last 40,000 Years of Biotic Change. University of Arizona Press, Tucson.
Author: Robert Bezy
Originally published in the Sonoran Herpetologist 2010 23(10):136-142.
For additional information on this species, please see the following volume and pages in the Sonoran Herpetologist: 2005 May:54-55.[/vc_column_text][/vc_column][vc_column width=”1/6″][/vc_column][/vc_row][vc_row][vc_column][gap size=”30px” id=”” class=”” style=””][/vc_column][/vc_row]